Polyethylene is produced by taking methane gas and converting it into ethylene and then applying the necessary heat and pressure to change it into polyethylene (Figure 1-1). (Lester H. Gabriel, n.d.)
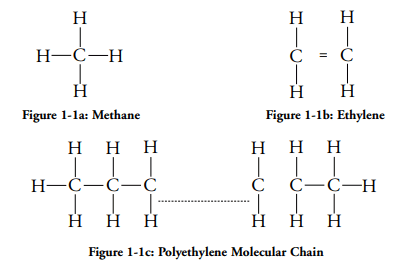
Figure 1-1 (Lester H. Gabriel, n.d.)
Properties of HDPE include: “Flexible, translucent/waxy, weatherproof, good low temperature toughness (to -60’C), easy to process by most methods, low cost, good chemical resistance.” (British Plastics Federation, n.d.)
Physical Properties:
Tensile Strength 0.20 – 0.40 N/mm²
Notched Impact Strength no break KJ/m²
Thermal Coefficient of expansion 100 – 220 x 10-6
Max Continued Use Temp 65 ‘C
Density 0.944 – 0.965 g/cm3 (British Plastics Federation, n.d.)
The atomic structure of HDPE is a hydrogen saturated carbon chain as shown in figure 1-1c. Its microstructure, from a brittle fracture compared to the ductile fracture in a sample of LDPE (Low-Density Polyethylene) is shown in figure 1-2.

Figure 1-2 (Noto, n.d.)
You can clearly see the bulbus tips of the fibres protruding from the HDPE’s surface, forming short stubs, while the LDPE has long globular fibres stretching from its surface in a much sparser spread than that of the HDPE. The micrographs in figure 1-2 have a magnification factor of 7000, which is in this case enough for all pertinent observations to be made.
While HDPE is an extremely versatile polymer, it is valuable to note that its most prevalent characteristic is its impressive strength to density ratio. This ratio is what makes HDPE standout from other polymers, especially LDPE which has a very similar density, but due to LDPE’s excessive branching it has a much lower tensile strength and weaker intermolecular forces than HDPE. (Negin Boostan Pardis Company, n.d.)